what is the maximum wavelegth a a photon can have and still be able to ionize a metal if
Learning Objectives
By the end of this department, y'all will exist able to:
- Explain the relationship betwixt the energy of a photon in joules or electron volts and its wavelength or frequency.
- Summate the number of photons per second emitted by a monochromatic source of specific wavelength and power.
Ionizing Radiation
A photon is a quantum of EM radiation. Its energy is given by Due east =hf and is related to the frequency f and wavelength λ of the radiation by
[latex]\displaystyle{E}=hf=\frac{hc}{\lambda}\text{(energy of a photon)}\\[/latex],
where Due east is the energy of a single photon and c is the speed of light. When working with small systems, energy in eV is often useful. Note that Planck's constant in these units is h = 4.xiv × ten−fifteen eV · s.
Since many wavelengths are stated in nanometers (nm), it is as well useful to know thathc= 1240 eV · nm.
These will make many calculations a little easier.
All EM radiation is composed of photons. Figure 1 shows various divisions of the EM spectrum plotted against wavelength, frequency, and photon free energy. Previously in this volume, photon characteristics were alluded to in the discussion of some of the characteristics of UV, 10 rays, and γ rays, the first of which start with frequencies only above violet in the visible spectrum. Information technology was noted that these types of EM radiation accept characteristics much different than visible light. We can at present see that such properties ascend because photon energy is larger at high frequencies.

Figure 1. The EM spectrum, showing major categories as a function of photon energy in eV, every bit well as wavelength and frequency. Certain characteristics of EM radiation are direct owing to photon free energy lonely.
| Table 1. Representative Energies for Submicroscopic Effects (Lodge of Magnitude Only) | |
|---|---|
| Rotational energies of molecules | x − 5 eV |
| Vibrational energies of molecules | 0.1 eV |
| Energy between outer electron shells in atoms | i eV |
| Bounden energy of a weakly bound molecule | 1 eV |
| Energy of red light | 2 eV |
| Binding free energy of a tightly bound molecule | x eV |
| Energy to ionize atom or molecule | 10 to 1000 eV |
Photons human activity as private quanta and interact with individual electrons, atoms, molecules, then on. The free energy a photon carries is, thus, crucial to the furnishings it has. Table i lists representative submicroscopic energies in eV. When we compare photon energies from the EM spectrum in Figure 1 with energies in the table, we can see how effects vary with the type of EM radiation.

Figure 2. One of the kickoff ten-ray images, taken by Röentgen himself. The hand belongs to Bertha Röentgen, his wife. (credit: Wilhelm Conrad Röntgen, via Wikimedia Eatables)
Gamma rays, a class of nuclear and cosmic EM radiations, tin accept the highest frequencies and, hence, the highest photon energies in the EM spectrum. For example, a γ-ray photon with f = 1021 Hz has an energy E =hf = 6.63 × 10−xiii J = four.14 MeV. This is sufficient energy to ionize thousands of atoms and molecules, since only 10 to 1000 eV are needed per ionization. In fact, γ rays are one blazon of ionizing radiation, every bit are ten rays and UV, because they produce ionization in materials that blot them. Because and so much ionization can be produced, a single γ-ray photon can cause significant damage to biological tissue, killing cells or damaging their ability to properly reproduce. When cell reproduction is disrupted, the result can be cancer, 1 of the known effects of exposure to ionizing radiation. Since cancer cells are rapidly reproducing, they are exceptionally sensitive to the disruption produced by ionizing radiation. This means that ionizing radiation has positive uses in cancer treatment as well equally risks in producing cancer.
High photon energy also enables γ rays to penetrate materials, since a collision with a single atom or molecule is unlikely to absorb all the γ ray'south energy. This can make γ rays useful as a probe, and they are sometimes used in medical imaging. x rays, equally yous can see in Figure 1, overlap with the low-frequency end of the γ ray range. Since x rays have energies of keV and up, individual 10-ray photons also can produce large amounts of ionization. At lower photon energies, x rays are non as penetrating as γ rays and are slightly less chancy. X rays are platonic for medical imaging, their most common use, and a fact that was recognized immediately upon their discovery in 1895 by the German physicist W. C. Roentgen (1845–1923). (Run across Figure 2.) Within one year of their discovery, 10 rays (for a time chosen Roentgen rays) were used for medical diagnostics. Roentgen received the 1901 Nobel Prize for the discovery of x rays.
Making Connections: Conservation of Energy
Once again, nosotros find that conservation of energy allows us to consider the initial and concluding forms that energy takes, without having to make detailed calculations of the intermediate steps. Example 1 is solved by considering simply the initial and terminal forms of energy.

Figure 3. Ten rays are produced when energetic electrons strike the copper anode of this cathode ray tube (CRT). Electrons (shown here as dissever particles) interact individually with the material they strike, sometimes producing photons of EM radiation.
While γ rays originate in nuclear decay, x rays are produced by the procedure shown in Effigy 3. Electrons ejected by thermal agitation from a hot filament in a vacuum tube are accelerated through a high voltage, gaining kinetic energy from the electrical potential free energy. When they strike the anode, the electrons convert their kinetic energy to a variety of forms, including thermal energy. But since an accelerated charge radiates EM waves, and since the electrons act individually, photons are also produced. Some of these x-ray photons obtain the kinetic energy of the electron. The accelerated electrons originate at the cathode, so such a tube is called a cathode ray tube (CRT), and diverse versions of them are plant in older TV and computer screens as well as in 10-ray machines.
Example 1. 10-ray Photon Energy and Ten-ray Tube Voltage
Observe the maximum energy in eV of an ten-ray photon produced by electrons accelerated through a potential difference of 50.0 kV in a CRT like the i in Figure iii.
Strategy
Electrons tin give all of their kinetic energy to a single photon when they strike the anode of a CRT. (This is something similar the photoelectric effect in contrary.) The kinetic energy of the electron comes from electrical potential energy. Thus we can but equate the maximum photon energy to the electrical potential energy—that is, hf=qV. (We do not take to summate each step from outset to cease if nosotros know that all of the starting energy qV is converted to the final form hf.)
Solution
The maximum photon energy is hf =qV, where q is the charge of the electron and 5 is the accelerating voltage. Thus,hf = (1.sixty × 10−xix C)(50.0 × x3 V).
From the definition of the electron volt, we know ane eV = 1.60 × 10−19 J, where 1 J = ane C ⋅ V. Gathering factors and converting free energy to eV yields
[latex]\displaystyle{hf}=\left(fifty.0\times10^iii\right)\left(ane.60\times10^{-19}\text{ C}\cdot\text{ V}\right)\left(\frac{1\text{ eV}}{1.60\times^{-19}\text{ C}\cdot\text{ 5}}\right)\\[/latex]
[latex]\displaystyle\qquad=\left(50.0\times10^iii\right)\left(one\text{ eV}\right)=50.0\text{ keV}\\[/latex]
Discussion
This example produces a upshot that tin be applied to many similar situations. If you accelerate a single elementary accuse, similar that of an electron, through a potential given in volts, so its energy in eV has the aforementioned numerical value. Thus a 50.0-kV potential generates 50.0 keV electrons, which in turn can produce photons with a maximum energy of 50 keV. Similarly, a 100-kV potential in an ten-ray tube tin can generate up to 100-keV x-ray photons. Many x-ray tubes have adjustable voltages and then that various free energy ten rays with differing energies, and therefore differing abilities to penetrate, can be generated.
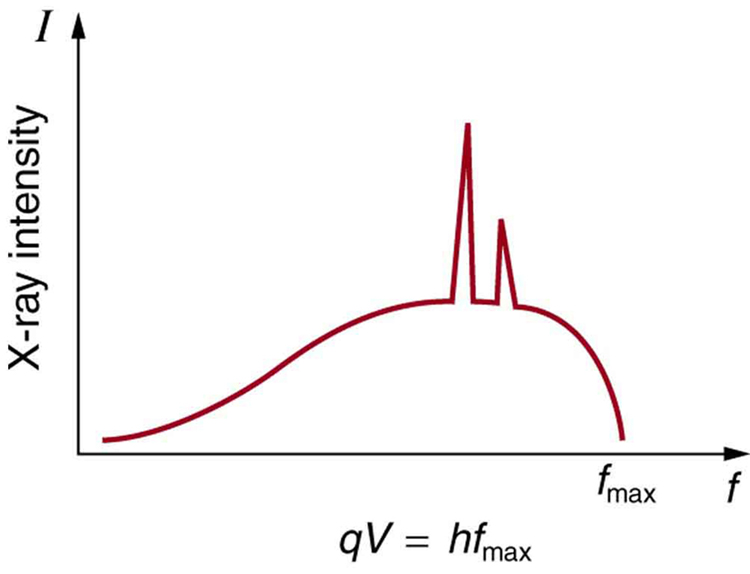
Figure 4. 10-ray spectrum obtained when energetic electrons strike a material. The smooth office of the spectrum is bremsstrahlung, while the peaks are characteristic of the anode material. Both are diminutive processes that produce energetic photons known as x-ray photons.
Effigy 4 shows the spectrum of 10 rays obtained from an ten-ray tube. In that location are ii distinct features to the spectrum. Beginning, the smooth distribution results from electrons being decelerated in the anode material. A curve similar this is obtained past detecting many photons, and it is apparent that the maximum energy is unlikely. This decelerating process produces radiation that is chosen bremsstrahlung (German for braking radiation). The second feature is the existence of sharp peaks in the spectrum; these are called characteristic 10 rays, since they are characteristic of the anode material. Characteristic x rays come from atomic excitations unique to a given type of anode material. They are akin to lines in diminutive spectra, implying the energy levels of atoms are quantized. Phenomena such as discrete diminutive spectra and feature 10 rays are explored further in Atomic Physics.
Ultraviolet radiation (approximately 4 eV to 300 eV) overlaps with the low terminate of the energy range of 10 rays, simply UV is typically lower in energy. UV comes from the de-excitation of atoms that may exist part of a hot solid or gas. These atoms tin be given energy that they after release every bit UV by numerous processes, including electrical discharge, nuclear explosion, thermal agitation, and exposure to x rays. A UV photon has sufficient energy to ionize atoms and molecules, which makes its effects different from those of visible calorie-free. UV thus has some of the same biological furnishings as γ rays and x rays. For instance, it tin crusade skin cancer and is used as a sterilizer. The major difference is that several UV photons are required to disrupt cell reproduction or impale a bacterium, whereas single γ-ray and X-ray photons can practice the same impairment. Just since UV does have the energy to alter molecules, information technology tin can do what visible light cannot. One of the beneficial aspects of UV is that it triggers the product of vitamin D in the peel, whereas visible light has insufficient energy per photon to change the molecules that trigger this production. Infantile jaundice is treated by exposing the baby to UV (with middle protection), called phototherapy, the beneficial furnishings of which are thought to be related to its ability to aid prevent the buildup of potentially toxic bilirubin in the blood.
Case ii. Photon Free energy and Effects for UV
Short-wavelength UV is sometimes called vacuum UV, because it is strongly absorbed by air and must exist studied in a vacuum. Calculate the photon energy in eV for 100-nm vacuum UV, and approximate the number of molecules it could ionize or intermission autonomously.
Strategy
Using the equation Due east =hf and advisable constants, we can find the photon energy and compare it with energy information in Table 1.
Solution
The energy of a photon is given by
[latex]East=hf=\frac{hc}{\lambda}\\[/latex].
Using hc = 1240 eV · nm, we find that
[latex]E=hf=\frac{hc}{\lambda}=\frac{1240\text{ eV }\cdot\text{ nm}}{100\text{ nm}}=12.4\text{ eV}\\[/latex].
Discussion
According to Tabular array one, this photon energy might be able to ionize an atom or molecule, and it is about what is needed to intermission upwardly a tightly jump molecule, since they are bound past approximately 10 eV. This photon energy could destroy well-nigh a dozen weakly bound molecules. Because of its high photon energy, UV disrupts atoms and molecules it interacts with. One practiced effect is that all but the longest-wavelength UV is strongly captivated and is hands blocked by sunglasses. In fact, most of the Sun'southward UV is absorbed by a thin layer of ozone in the upper atmosphere, protecting sensitive organisms on Earth. Damage to our ozone layer by the addition of such chemicals as CFCs has reduced this protection for u.s..
Visible Light
The range of photon energies for visible light from blood-red to violet is 1.63 to 3.26 eV, respectively (left for this affiliate's Problems and Exercises to verify). These energies are on the order of those between outer electron shells in atoms and molecules. This means that these photons tin can be captivated by atoms and molecules. A single photon can actually stimulate the retina, for example, by altering a receptor molecule that then triggers a nerve impulse. Photons can be absorbed or emitted merely by atoms and molecules that have precisely the correct quantized energy pace to do so. For example, if a red photon of frequency f encounters a molecule that has an free energy step, ΔEast, equal to hf, then the photon can be absorbed. Violet flowers absorb scarlet and reverberate violet; this implies there is no energy step betwixt levels in the receptor molecule equal to the violet photon's energy, but there is an energy step for the cherry-red.

Figure 5. Why practice the reds, yellows, and greens fade before the blues and violets when exposed to the Sun, every bit with this affiche? The answer is related to photon energy. (credit: Deb Collins, Flickr)
In that location are some noticeable differences in the characteristics of light betwixt the two ends of the visible spectrum that are due to photon energies. Red light has insufficient photon free energy to expose about black-and-white motion picture, and it is thus used to illuminate darkrooms where such moving-picture show is developed. Since violet light has a higher photon free energy, dyes that blot violet tend to fade more quickly than those that practise not. (See Figure 5.) Take a look at some faded color posters in a storefront some time, and you will find that the dejection and violets are the terminal to fade. This is because other dyes, such as cerise and dark-green dyes, absorb bluish and violet photons, the college energies of which break up their weakly jump molecules. (Complex molecules such as those in dyes and Dna tend to be weakly leap.) Blue and violet dyes reverberate those colors and, therefore, do not absorb these more energetic photons, thus suffering less molecular damage.
Transparent materials, such as some glasses, do not absorb any visible lite, because at that place is no energy stride in the atoms or molecules that could blot the light. Since individual photons interact with individual atoms, it is near impossible to have two photons absorbed simultaneously to reach a large energy step. Because of its lower photon energy, visible light tin sometimes pass through many kilometers of a substance, while higher frequencies like UV, x ray, and γ rays are absorbed, because they accept sufficient photon energy to ionize the material.
Example 3. How Many Photons per Second Does a Typical Lite Bulb Produce?
Assuming that 10.0% of a 100-W light bulb's free energy output is in the visible range (typical for incandescent bulbs) with an average wavelength of 580 nm, calculate the number of visible photons emitted per second.
Strategy
Ability is energy per unit of measurement time, and so if we can find the energy per photon, we tin determine the number of photons per second. This volition all-time be washed in joules, since power is given in watts, which are joules per 2d.
Solution
The power in visible low-cal product is x.0% of 100 W, or x.0 J/s. The energy of the average visible photon is found by substituting the given average wavelength into the formula [latex]Eastward=\frac{hc}{\lambda}\\[/latex].
This produces
[latex]\displaystyle{Due east}=\frac{\left(6.63\times10^{-34}\text{ J }\cdot\text{ s}\right)\left(3.00\times10^viii\text{ one thousand/southward}\right)}{580\times10^{-9}\text{ m}}=3.43\times10^{-19}\text{ J}\\[/latex]
The number of visible photons per second is thus
[latex]\displaystyle\text{photon/southward}=\frac{10.0\text{ J/s}}{3.42\times10^{-19}\text{ J/photon}}=2.92\times10^{19}\text{ photon/s}\\[/latex]
Discussion
This incredible number of photons per 2nd is verification that individual photons are insignificant in ordinary human experience. It is also a verification of the correspondence principle—on the macroscopic scale, quantization becomes essentially continuous or classical. Finally, at that place are and so many photons emitted by a 100-W lightbulb that information technology tin can be seen past the unaided eye many kilometers abroad.
Lower-Energy Photons
Infrared radiation (IR) has even lower photon energies than visible light and cannot significantly change atoms and molecules. IR tin be absorbed and emitted by atoms and molecules, particularly betwixt closely spaced states. IR is extremely strongly captivated by h2o, for example, because water molecules accept many states separated by energies on the gild of 10−5 eV to x−ii eV, well within the IR and microwave free energy ranges. This is why in the IR range, skin is virtually jet blackness, with an emissivity nigh i—there are many states in h2o molecules in the skin that can blot a large range of IR photon energies. Non all molecules have this holding. Air, for example, is nearly transparent to many IR frequencies.
Microwaves are the highest frequencies that can exist produced by electronic circuits, although they are also produced naturally. Thus microwaves are similar to IR but exercise not extend to as loftier frequencies. In that location are states in water and other molecules that accept the aforementioned frequency and energy as microwaves, typically about x−5 eV. This is i reason why food absorbs microwaves more than strongly than many other materials, making microwave ovens an efficient style of putting free energy straight into food.
Photon energies for both IR and microwaves are so low that huge numbers of photons are involved in any meaning free energy transfer past IR or microwaves (such as warming yourself with a heat lamp or cooking pizza in the microwave). Visible light, IR, microwaves, and all lower frequencies cannot produce ionization with single photons and do non unremarkably have the hazards of college frequencies. When visible, IR, or microwave radiations is chancy, such every bit the inducement of cataracts past microwaves, the hazard is due to huge numbers of photons acting together (non to an accumulation of photons, such as sterilization by weak UV). The negative effects of visible, IR, or microwave radiation tin be thermal effects, which could be produced past whatsoever oestrus source. But i difference is that at very high intensity, strong electric and magnetic fields tin exist produced by photons acting together. Such electromagnetic fields (EMF) can actually ionize materials.
Misconception Warning: High-Voltage Power Lines
Although some people recall that living near high-voltage power lines is hazardous to one'due south health, ongoing studies of the transient field effects produced past these lines testify their strengths to be insufficient to cause damage. Demographic studies also fail to prove significant correlation of ill effects with high-voltage power lines. The American Concrete Club issued a written report over ten years ago on power-line fields, which concluded that the scientific literature and reviews of panels testify no consistent, significant link between cancer and power-line fields. They also felt that the "diversion of resource to eliminate a threat which has no persuasive scientific footing is disturbing."
Information technology is nigh impossible to notice individual photons having frequencies below microwave frequencies, because of their depression photon energy. But the photons are at that place. A continuous EM wave can be modeled as photons. At low frequencies, EM waves are by and large treated as time- and position-varying electric and magnetic fields with no discernible quantization. This is another example of the correspondence principle in situations involving huge numbers of photons.
PhET Explorations: Color Vision
Make a whole rainbow past mixing red, green, and blue light. Change the wavelength of a monochromatic beam or filter white light. View the light as a solid beam, or see the individual photons.
Click to run the simulation.
Section Summary
- Photon energy is responsible for many characteristics of EM radiation, existence particularly noticeable at loftier frequencies.
- Photons accept both wave and particle characteristics.
Conceptual Questions
- Why are UV, ten rays, and γ rays called ionizing radiations?
- How tin can treating food with ionizing radiation help continue it from spoiling? UV is not very penetrating. What else could be used?
- Some television receiver tubes are CRTs. They utilise an approximately 30-kV accelerating potential to send electrons to the screen, where the electrons stimulate phosphors to emit the light that forms the pictures we picket. Would y'all await x rays also to be created?
- Tanning salons use "prophylactic" UV with a longer wavelength than some of the UV in sunlight. This "condom" UV has enough photon free energy to trigger the tanning mechanism. Is information technology likely to be able to cause jail cell damage and induce cancer with prolonged exposure?
- Your pupils dilate when visible calorie-free intensity is reduced. Does wearing sunglasses that lack UV blockers increase or subtract the UV hazard to your optics? Explain.
- One could feel heat transfer in the course of infrared radiation from a large nuclear bomb detonated in the temper 75 km from yous. However, none of the profusely emitted x rays or [latex]\gamma\\[/latex] rays reaches you. Explicate.
- Can a single microwave photon cause prison cell harm? Explicate.
- In an x-ray tube, the maximum photon energy is given past hf = qV. Would it be technically more correct to say hf= qV+ BE, where Be is the binding energy of electrons in the target anode? Why isn't the energy stated the latter way?
Bug & Exercises
- What is the energy in joules and eV of a photon in a radio wave from an AM station that has a 1530-kHz broadcast frequency?
- (a) Find the free energy in joules and eV of photons in radio waves from an FM station that has a 90.0-MHz broadcast frequency. (b) What does this imply almost the number of photons per 2d that the radio station must broadcast?
- Calculate the frequency in hertz of a 1.00-MeV γ-ray photon.
- (a) What is the wavelength of a 1.00-eV photon? (b) Find its frequency in hertz. (c) Identify the type of EM radiations.
- Do the unit conversions necessary to show that hc = 1240 eV · nm, equally stated in the text.
- Confirm the statement in the text that the range of photon energies for visible lite is 1.63 to 3.26 eV, given that the range of visible wavelengths is 380 to 760 nm.
- (a) Calculate the energy in eV of an IR photon of frequency ii.00 × 10thirteen Hz. (b) How many of these photons would need to be absorbed simultaneously by a tightly spring molecule to interruption it autonomously? (c) What is the energy in eV of a γ ray of frequency 3.00 × 10xx Hz? (d) How many tightly leap molecules could a single such γ ray intermission apart?
- Prove that, to 3-digit accuracy, h= 4.fourteen × 10−15 eV · s, as stated in the text.
- (a) What is the maximum free energy in eV of photons produced in a CRT using a 25.0-kV accelerating potential, such every bit a color TV? (b) What is their frequency?
- What is the accelerating voltage of an x-ray tube that produces x rays with a shortest wavelength of 0.0103 nm?
- (a) What is the ratio of ability outputs by ii microwave ovens having frequencies of 950 and 2560 MHz, if they emit the aforementioned number of photons per 2d? (b) What is the ratio of photons per second if they take the same power output?
- How many photons per 2nd are emitted by the antenna of a microwave oven, if its power output is one.00 kW at a frequency of 2560 MHz?
- Some satellites use nuclear power. (a) If such a satellite emits a 1.00-W flux of γ rays having an average energy of 0.500 MeV, how many are emitted per 2d? (b) Theseγ rays bear upon other satellites. How far away must some other satellite exist to only receive 1γ ray per 2nd per square meter?
- (a) If the ability output of a 650-kHz radio station is 50.0 kW, how many photons per 2d are produced? (b) If the radio waves are broadcast uniformly in all directions, find the number of photons per second per foursquare meter at a distance of 100 km. Assume no reflection from the ground or assimilation by the air.
- How many 10-ray photons per second are created by an 10-ray tube that produces a flux of ten rays having a power of 1.00 West? Assume the average energy per photon is 75.0 keV.
- (a) How far abroad must you exist from a 650-kHz radio station with power 50.0 kW for there to be only i photon per second per foursquare meter? Assume no reflections or absorption, equally if you were in deep outer space. (b) Hash out the implications for detecting intelligent life in other solar systems by detecting their radio broadcasts.
- Assuming that 10.0% of a 100-W low-cal seedling's energy output is in the visible range (typical for incandescent bulbs) with an average wavelength of 580 nm, and that the photons spread out uniformly and are not absorbed by the atmosphere, how far away would yous be if 500 photons per 2d enter the 3.00-mm diameter educatee of your eye? (This number easily stimulates the retina.)
- Construct Your Own Trouble. Consider a laser pen. Construct a problem in which you summate the number of photons per second emitted by the pen. Among the things to exist considered are the light amplification by stimulated emission of radiation pen's wavelength and power output. Your teacher may also wish for you lot to make up one's mind the minimum diffraction spreading in the beam and the number of photons per square centimeter the pen can project at some large distance. In this latter case, you volition also need to consider the output size of the laser beam, the distance to the object being illuminated, and any assimilation or scattering forth the fashion.
Glossary
gamma ray: also γ-ray; highest-energy photon in the EM spectrum
ionizing radiation: radiation that ionizes materials that absorb it
ten ray: EM photon between γ-ray and UV in energy
bremsstrahlung: German for braking radiations; produced when electrons are decelerated
characteristic x rays: x rays whose energy depends on the material they were produced in
ultraviolet radiation: UV; ionizing photons slightly more energetic than violet low-cal
visible light: the range of photon energies the human eye can notice
infrared radiation: photons with energies slightly less than ruddy light
microwaves: photons with wavelengths on the guild of a micron (μm)
Selected Solutions to Problems & Exercises
1. vi.34 × 10−9 eV, ane.01 × 10−27 J
three. 2.42 × 1020 Hz
5. [latex]\begin{array}{lll}\text{hc}& =& \left(\text{6.62607}\times {\text{10}}^{-34}\text{ J}\cdot \text{ s}\right)\left(\text{two.99792}\times {\text{10}}^{8}\text{ grand/s}\right)\left(\frac{{\text{10}}^{9}\text{ nm}}{1\text{ m}}\right)\left(\frac{i.00000\text{ eV}}{ane.60218\times{10}^{-19}\text{ J}}\right)\\\text{ }& =&1239.84\text{ eV}\cdot \text{nm}\\\text{ }&\approx&1240\text{ eV}\cdot\text{ nm}\cease{array}\\[/latex]
7. (a) 0.0829 eV; (b) 121; (c) 1.24 MeV; (d) 1.24 × 105
9. (a) 25.0 × 103 eV; (b) vi.04 × 1018 Hz
xi. (a) 2.69; (b) 0.371
13. (a) 1.25 × 1013 photons/s; (b) 997 km
15. viii.33 × xthirteen photons/southward
17. 181 km
carpentervidereps.blogspot.com
Source: https://courses.lumenlearning.com/physics/chapter/29-3-photon-energies-and-the-electromagnetic-spectrum/
Post a Comment for "what is the maximum wavelegth a a photon can have and still be able to ionize a metal if"